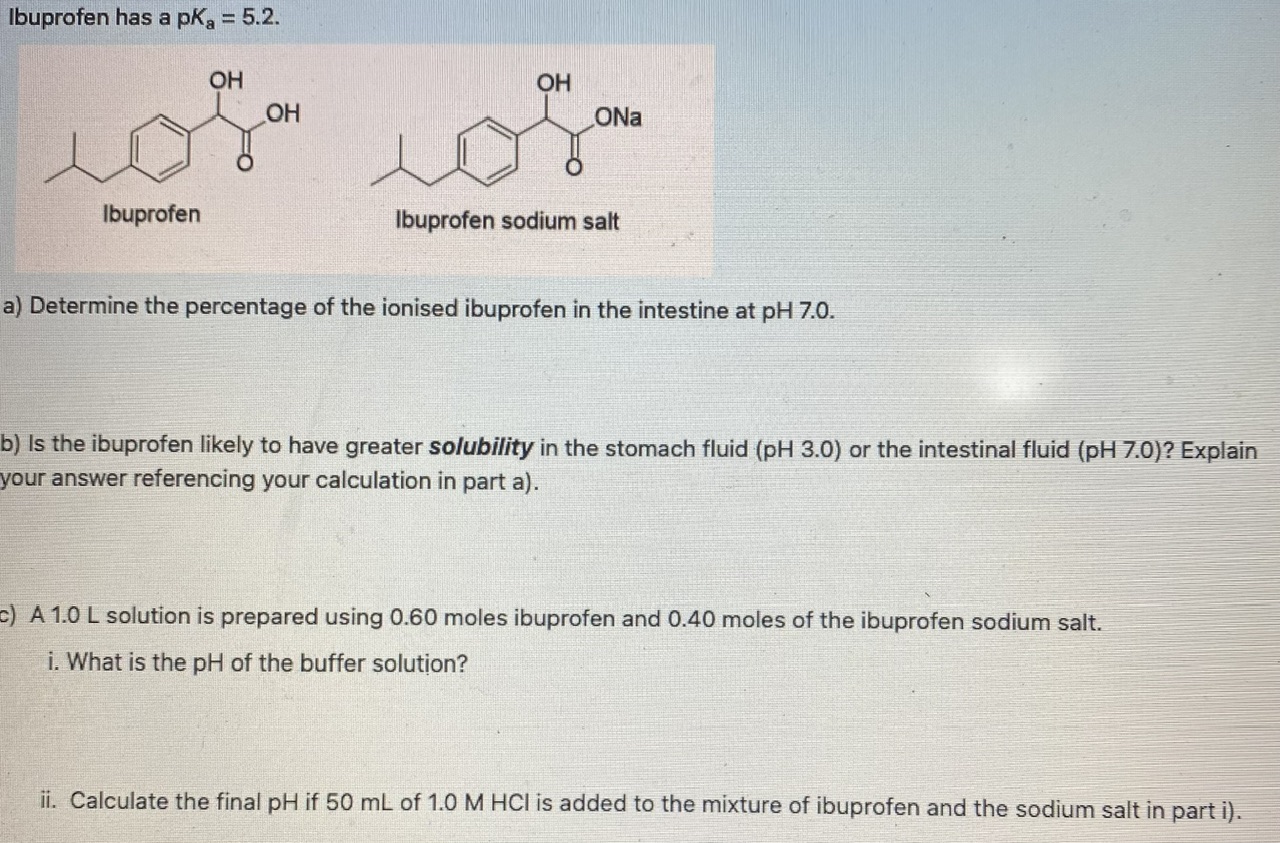
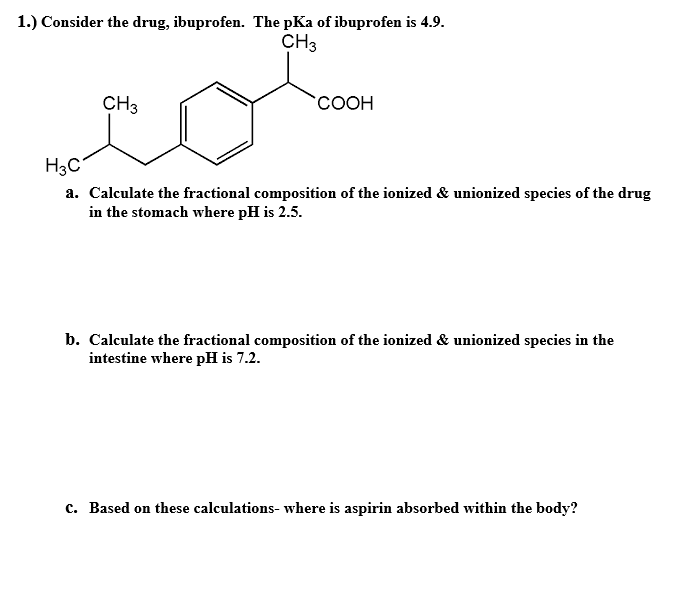
Nanomaterials | Free Full-Text | Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures
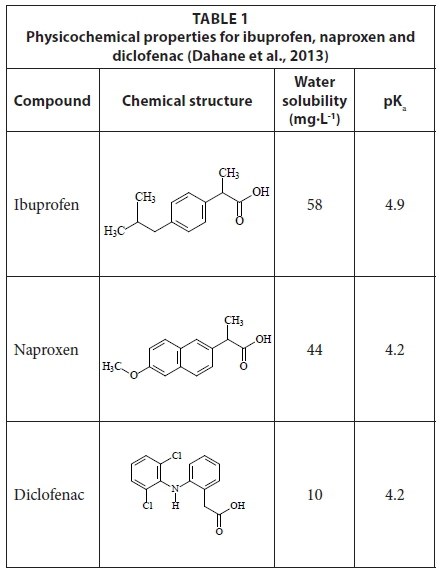
Simultaneous determination of naproxen, ibuprofen and diclofenac in wastewater using solid-phase extraction with high performance liquid chromatography

Effect of Water pH on the Uptake of Acidic (Ibuprofen) and Basic (Propranolol) Drugs in a Fish Gill Cell Culture Model | Environmental Science & Technology
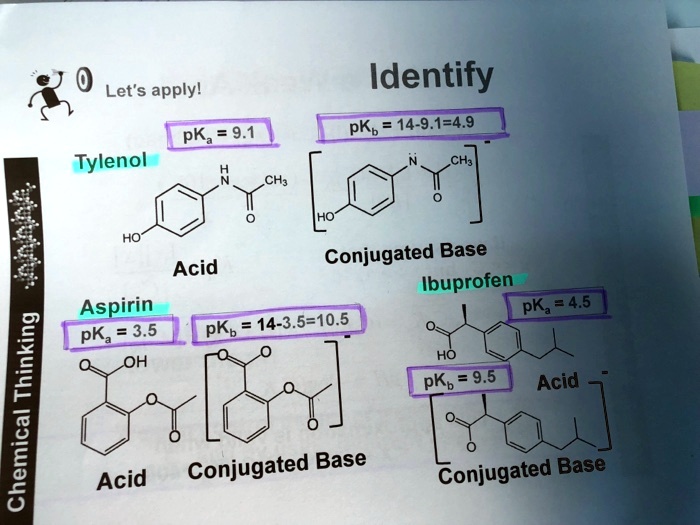
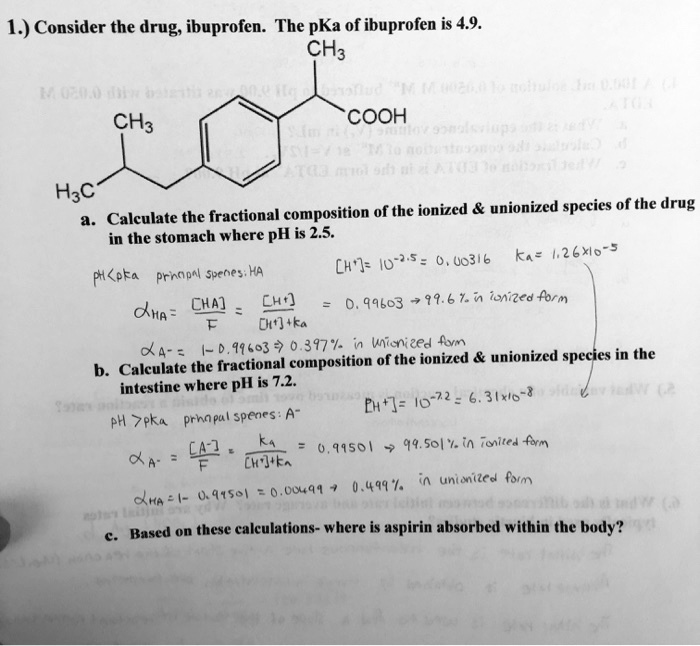
SOLVED: Let's apply. Identify pKb = 14 - 9.1 = 4.9. pKa = 9.1. Tylenol. CH3. CH3. HO. Conjugated Base Acid Ibuprofen Aspirin pKa = 4.5 pKb = 14 - 3.5 - 10.5 pKa OH HO pKb = 9.5 Acid. 1 7 Acid Conjugated Base Conjugated Base.
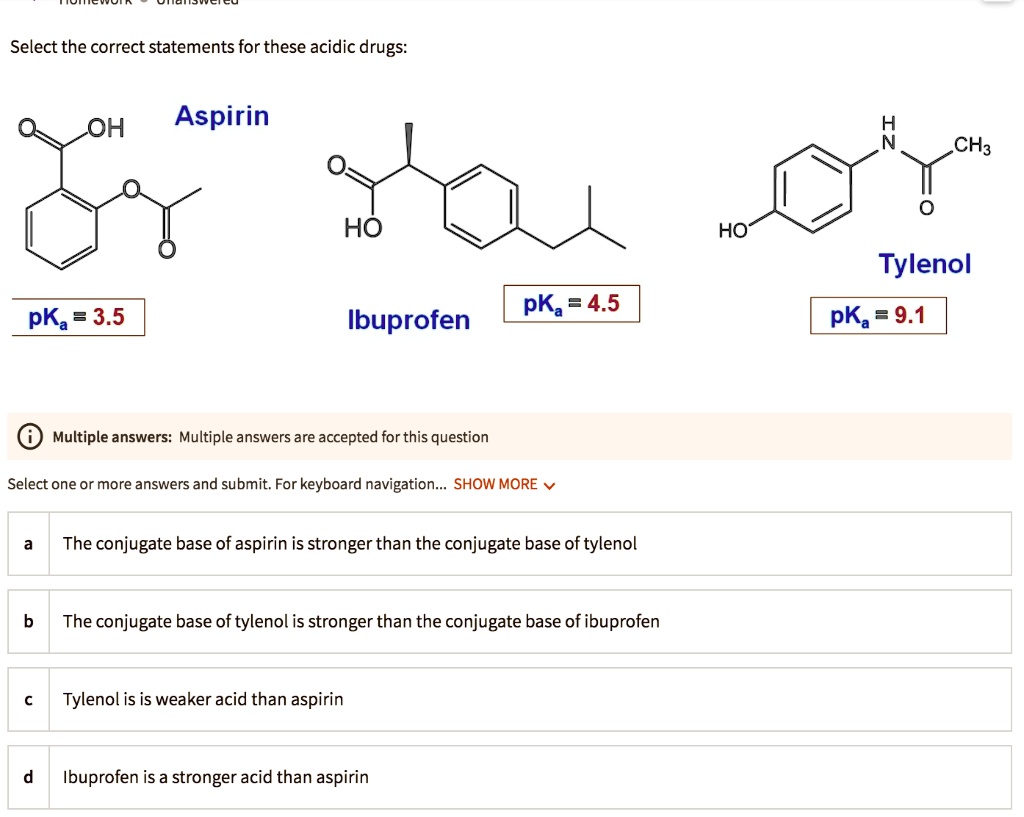
SOLVED: Select the correct statements for these acidic drugs: OH Aspirin HO HO Tylenol pKa 9.1 pKa =4.5 pKa 3.5 Ibuprofen Multiple answers: Multiple answers are accepted for this question Select one

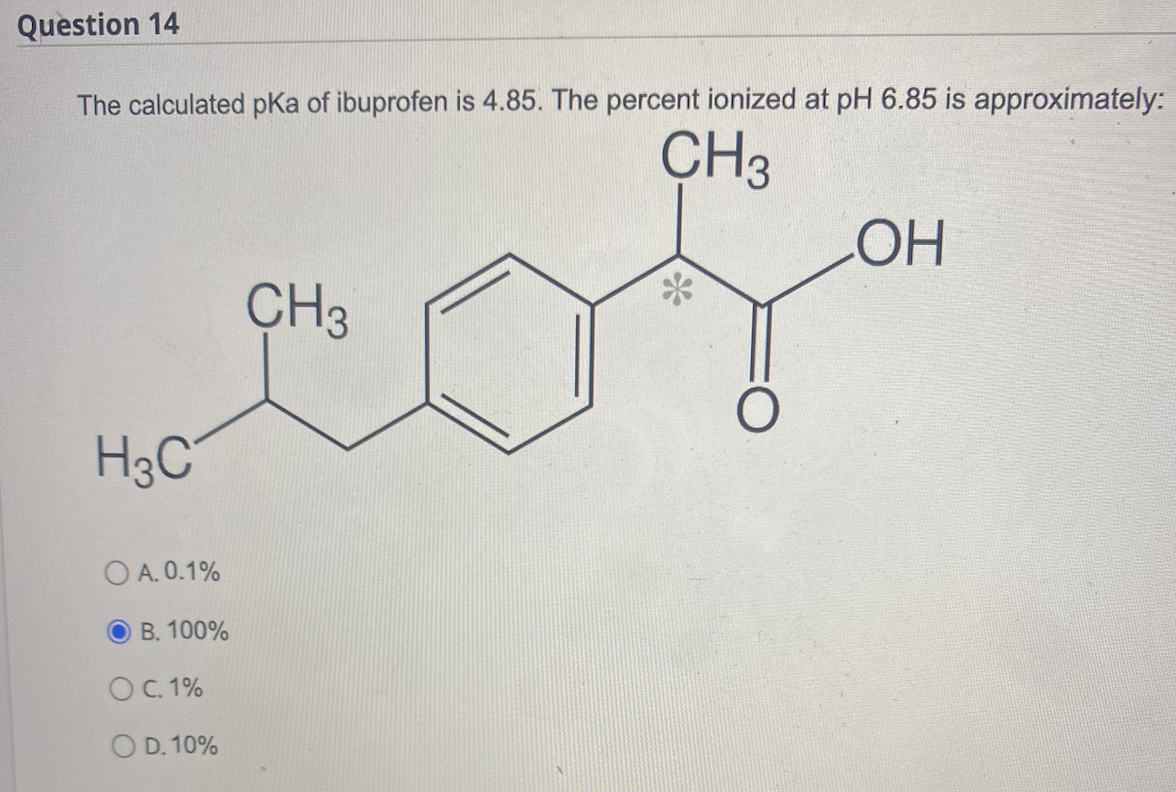
Figure1: Ibuprofen with a molecular weight of 206.3, pKa of 4.9, and... | Download Scientific Diagram

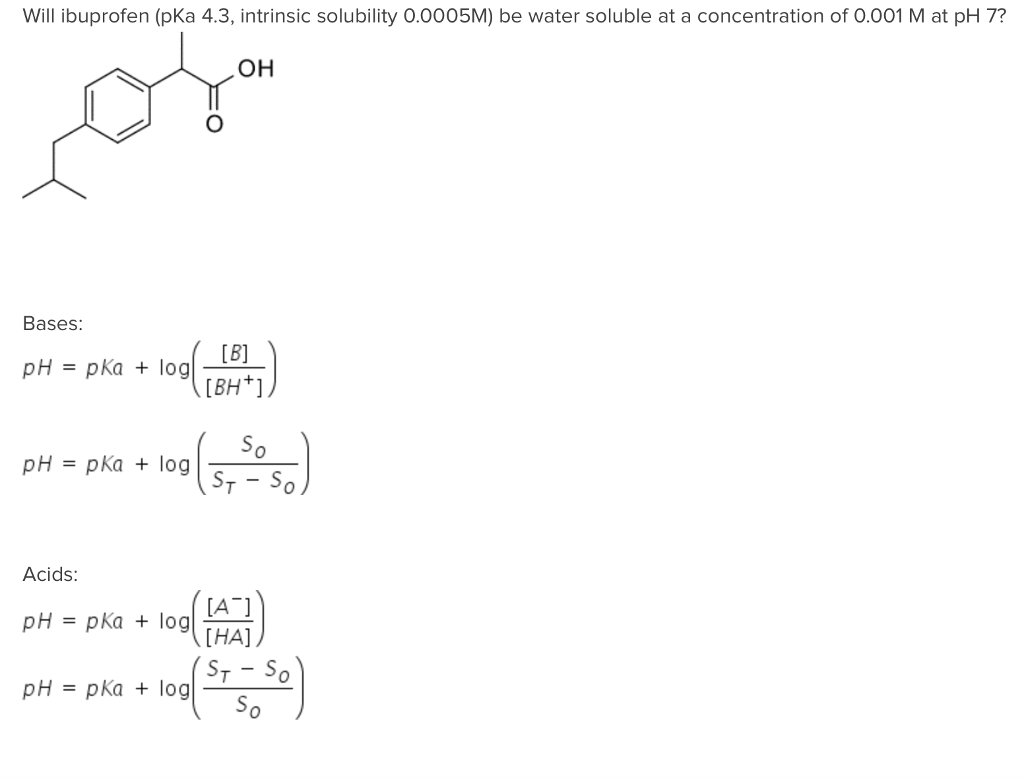
Ibuprofen: water affinity, effect of acidic pH and resonance structure:... | Download Scientific Diagram

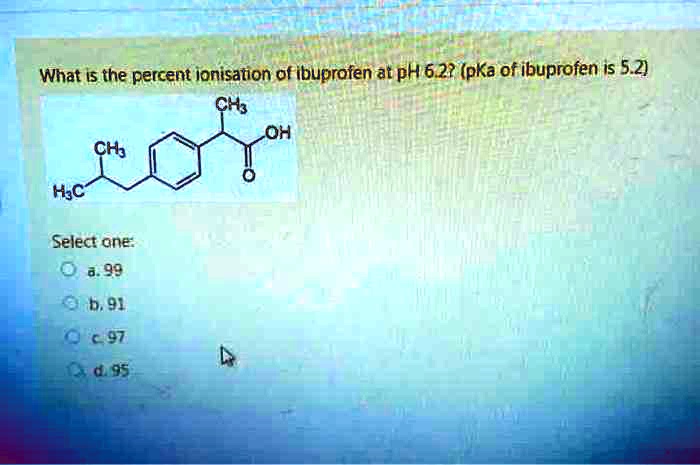
Effects of pH, dissolved organic matter, and salinity on ibuprofen sorption on sediment | Environmental Science and Pollution Research
![PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/bd825006414bdf48d3e25685d998bf571ff563c7/2-Figure1-1.png)
PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar